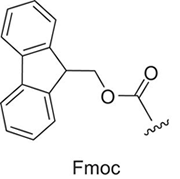
Peptide synthesis and choice of protection groups
In Fmoc peptide chemistry, the deprotection of Fmoc is accomplished in mild basic solution (piperidine), and the side-chain protecting groups have to be stable under this condition. They must however, be easily cleaved under the final acid resin cleavage condition. The most commonly used side-chain protecting groups in Fmoc peptide chemistry are the following:
Arg(Pbf),Asn(Trt),Asp(OtBu),Cys(Trt),Glu(OtBu),Gln(Trt),His(Trt),Lys(Boc),Ser(tBu),Thr(tBu),Trp(Boc), Tyr(tBu). When making modified peptides, some protecting groups need to be removed individually without affecting others. Some of these special protection groups are: Lys(Dde),Lys(Mmt),Asp(ODmab),Cys(Acm).
The N-terminal amino protecting group Fmoc can be easily removed by 20-30% Piperidine in DMF. The reaction is very fast, usually reaches completion within 4-10 min. Adding 0.1M HOBt to the deprotecting mixture will suppress the side reaction of the aspartimide formation when Asp is in the peptide sequence. The Fmoc deprotection can be sluggish when the secondary structure formation of the peptide is high. In this case, the first choice would be to extend the reaction time. When such action is not sufficient, the stronger base DBU can be used together with piperidine.